How do holistic and functional medicine practitioners feel about the Dietary Supplements Health and Education Act (DSHEA) and the peculiar language games it obliges supplement makers to play?
This Spring, Holistic Primary Care sought to find out. Our 2019 practitioner survey, which fielded in April, included a series of questions about the supplement industry and the key regulations that govern it. The findings are quite interesting.
We obtained responses from 355 practitioners, 32% of whom are MDs, with 15% being naturopaths; 10% osteopaths; 10% nurses; 7% chiropractors, and a number of acupuncturists, nutrition counselors, wellness coaches, and psychotherapists.
Women outnumbered men (68% to 32%), and most are in solo (51%) or small group practices of 2-10 practitioners (20%).
The survey included the following question:
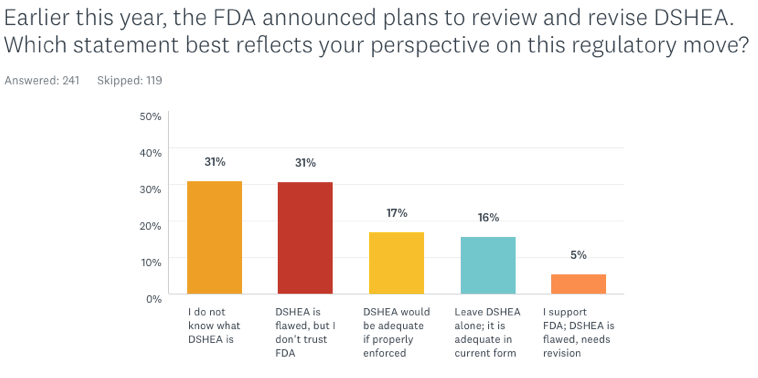
Earlier this year, the FDA announced plans to review and revise DSHEA, which has not been changed since it was first passed in 1994. Which statement best reflects your perspective on this regulatory move?
- DSHEA is fully adequate and effective to protect public safety. The FDA should leave it alone.
- DSHEA would be adequate if properly enforced. FDA should concentrate on better enforcement rather than revision
- DSHEA is flawed and needs major revision. I support the FDA’s intention to review and revise it
- DSHEA has problems, but I do not trust the FDA’s intention for revising it
- I do not know what DSHEA is
The first fact that jumps out from the data is that almost one-third of respondents (31%) do not know what DSHEA is. That number may seem high, but it’s actually an improvement over 2016 (the first year we asked questions about DSHEA) when 57% did recognize the acronym.
Only 16% of the respondents feel the law is adequate to protect public safety; another 17% believe that DSHEA would be adequate if properly enforced.
While 31% see DSHEA as problematic, they do not trust the FDA to revise it. Notably, only 5% of the respondents are fully supportive of the FDA’s intention to revise the regulation.
It is interesting that conventionally trained MDs tracked very closely with the general cohort in their attitudes about DSHEA: 39% don’t know what it is; 28% see the law as problematic but do not trust the FDA to revise it; 16% believe it would be adequate if properly enforced; and 11% view the law as fully adequate as it is. Only 6% of the MDs fully support the FDA’s intention to review and revise DSHEA.
Ambivalence Toward FDA, Industry
The verbatim comments are indicative of practitioners’ complex, sometimes conflicting views about both the supplement industry and the federal agency that is supposed to regulate it. One clinician wrote:
“I’m concerned on both ends of this dilemma. There needs to be some review and enforcement. However, I am not sure when it comes to pharmacy lobbyists among others and their influence on the FDA to limit less expensive preventative, supportive and/or treatment options.”
Another points out:
“FDA approved a lot of the conventional medicine, which is killing thousands of people every year or damaging their health even. For me FDA approved doesn’t mean it is good”
Our survey shows that practitioners are no fans of the “structure/function” based marketing language that DSHEA obliges supplement companies to use. Put another way, most Holistic Primary Care readers would like it if supplement companies could use truthful disease terminology when they communicate with medical audiences.
Need for Clear Communication
The survey asked:
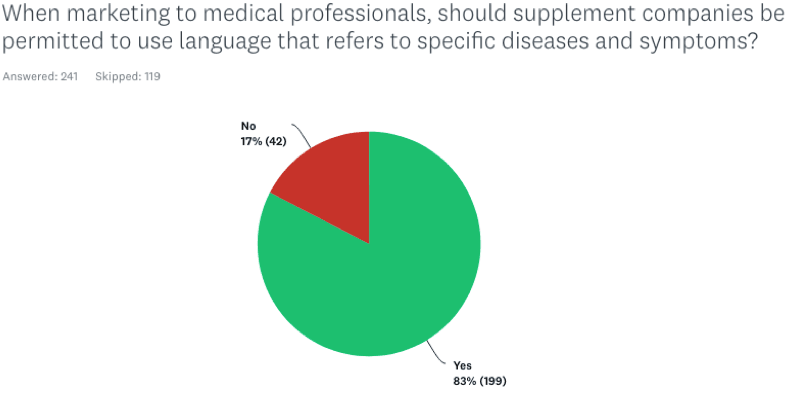
When marketing to medical professionals, should supplement companies be permitted to use language that refers to specific diseases and symptoms?
83% of the practitioners responded Yes; only 17% said No.
 Then, we asked:
Then, we asked:
When marketing to the general public, should supplement companies be permitted to use language that refers to specific diseases and symptoms?
Here the response was more subdued, with 39% indicating Yes, and 61% voting No. Again, the MDs tracked very closely with the general cohort.
In essence, the data suggest that holistic practitioners are comfortable with two-tiered communications, with greater latitude for disease claims on the practitioner side.
That’s not entirely surprising: until 1990s, when direct-to-consumer pharma advertising became widespread, physicians and patients dwelled in two vastly different information worlds, with little overlap between them.
Join the Dialog on DSHEA
Our survey population is a decidedly supplement-friendly cohort. Nearly all 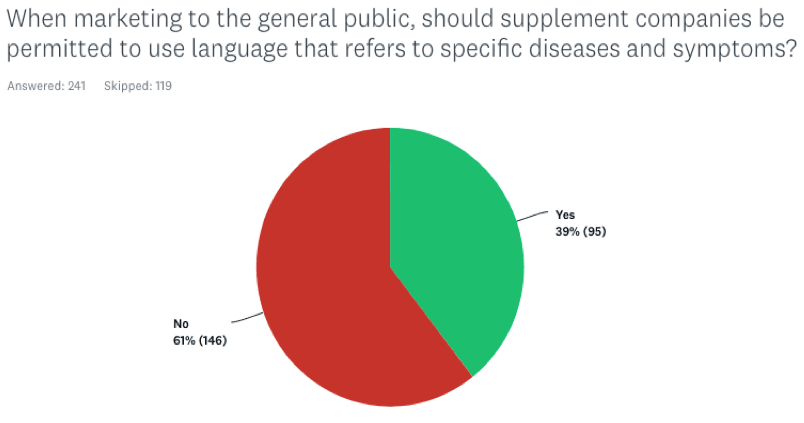 respondents (95%) take supplements themselves, and 66% dispense (ie, sell) them in their practices. Even among those who do not dispense, 93% recommend supplements to their patients. So, it is to be expected that there’s a lot of support for more open communication about supplements and their benefits. A more conventional cohort would likely be more wary.
respondents (95%) take supplements themselves, and 66% dispense (ie, sell) them in their practices. Even among those who do not dispense, 93% recommend supplements to their patients. So, it is to be expected that there’s a lot of support for more open communication about supplements and their benefits. A more conventional cohort would likely be more wary.
Still, the fact that more than 80% of our conventionally-trained MDs believe supplement makers should be able to use disease language in their medically-focused communications is highly significant.
It seems very clear that FDA intends to open DSHEA for review and revision over the next few years.
While it is very unlikely that “DSHEA 2.0” will permit supplement companies to make disease claims, the need for clear and truthful clinical communication should at least be part of the discussion. As one of our survey respondents noted:
“I would suggest adding practitioners who use these products to be on the advisory committees.”
The truth is, if you use or recommend supplements in your clinical practice, you are affected by DSHEA, and you will be affected by any changes in the law. Your needs and concerns as a clinician need to be part of the discussion.
The absence of medical perspectives was excusable in 1994, when DSHEA was drafted. In 2019, when thousands of practitioners are routinely using supplements, it is not.
END







